.
Acumen Pharmaceuticals is establishing an Alzheimers illness drug that it thinks offers benefits compared to other techniques– consisting of the recently authorized Biogen therapy. A small scientific trial is underway to possibly demonstrate those claims, and the company now has $160 million from its IPO to continue its research.
Acumen priced its shares at $16 each, the top end of the predicted $14 to $16 per share price range. Financiers showed enthusiasm for Acumens approach, as its shares opened at $25 each, more than 56% higher than the IPO price.
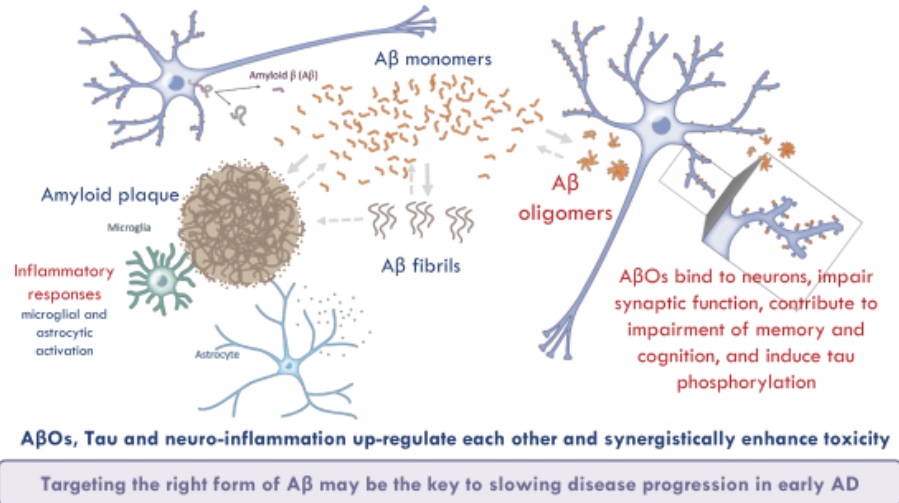
The research study of Acumen follows on the so-called amyloid hypothesis, which posits that Alzheimers disease comes from the build-up of beta amyloid protein into plaques in the brain. Drug designers have actually attended to these plaques with antibodies that aim to either break up the accumulation of the problem protein or target beta amyloid monomers, the type of the protein before it ends up being pathogenic, the company said in the IPO filing. The majority of those techniques have either stopped working to reveal efficacy or have been stymied by toxicity issues.
In a “improvement” of the amyloid hypothesis, Acumen states research has found a minimum of 3 various forms of beta amyloid in the body. Acumens research focuses on soluble beta amyloid oligomers (AbOs), the type of amyloid protein that has clustered together. In the IPO filing, the business stated it believes this type of amyloid is the most pathogenic and harmful form, triggering toxicity in synapses along with neurodegeneration. In animal research study, build-up of AbOs is related to the deterioration and loss of synapses, the formation of tangles of tau protein, and inflammation. Acumen adds that this type of amyloid is associated with behavioral flaws, consisting of learning and memory disability.
” In light of this evidence, we think that blocking the toxicity of [beta amyloid oligomers] is the most promising technique for the treatment of ADVERTISEMENT (Alzheimers disease), which led us to find and establish ACU193,” Acumen said in the IPO filing.
ACU193 is a monoclonal antibody offered as an intravenous infusion. The Acumen drug is developed to selectively bind to AbOs. While Biogens recently approved Aduhelm likewise targets AbOs along with beta amyloid plaques, Acumen believes its drug could offer a safety advantage. Swelling in the brain and tiny spots of bleeding are amongst the adverse effects risks listed for the Biogen drug. Due to those dangers, the FDA stated that clients must go through routine brain imaging to look for these issues. Acumen said that its antibody has actually been crafted to decrease its impact on immune cells. It is likewise created to prevent binding to amyloid on vascular cells. The company anticipates those functions will lower the incidence of problems associated with amyloid plaque-targeting treatments.
Acumen still requires to demonstrate its approach can work in human beings. A Phase 1 clinical trial began in April, intending to enlist 62 patients with mild dementia or mild cognitive impairment due to Alzheimers. In addition to examining the security and tolerability of the drug, the research study is likewise planned to show the mechanism of ACU193. Information are expected by the end of 2022.
Acumen incorporated in 1996 but ACU193 traces its origins to the labs of Merck, which in 2003 started the research study that led up to the antibody. That year, Acumen started an R&D partnership with the pharmaceutical giant focusing on Alzheimers disease immunotherapies, according to the IPO filing. ACU193 emerged as the lead product prospect from that research study, Merck chose to end the program in 2011 and turn its focus to a different drug prospect. Acumen then acquired the rights to the ended research, including ACU193.
Acumen is, so far, a one-drug company and little occurred with that one drug till relatively just recently. In 2018, Acumen raised its first institutional financing, which restored the companys research.
At the end of the first quarter of this year, Acumen reported having $41.4 million in cash. Acumen plans to spend $75 million to complete the Phase 1 test of ACU193. The company approximates that the cash will be adequate to support the company through 2023.
The research study of Acumen follows on the so-called amyloid hypothesis, which presumes that Alzheimers disease stems from the accumulation of beta amyloid protein into plaques in the brain. In a “refinement” of the amyloid hypothesis, Acumen says research study has found at least three different forms of beta amyloid in the body. Acumens research focuses on soluble beta amyloid oligomers (AbOs), the kind of amyloid protein that has clustered together. Acumen includes that this form of amyloid is associated with behavioral problems, including learning and memory impairment.
While Biogens recently authorized Aduhelm likewise targets AbOs as well as beta amyloid plaques, Acumen thinks its drug might provide a safety benefit.