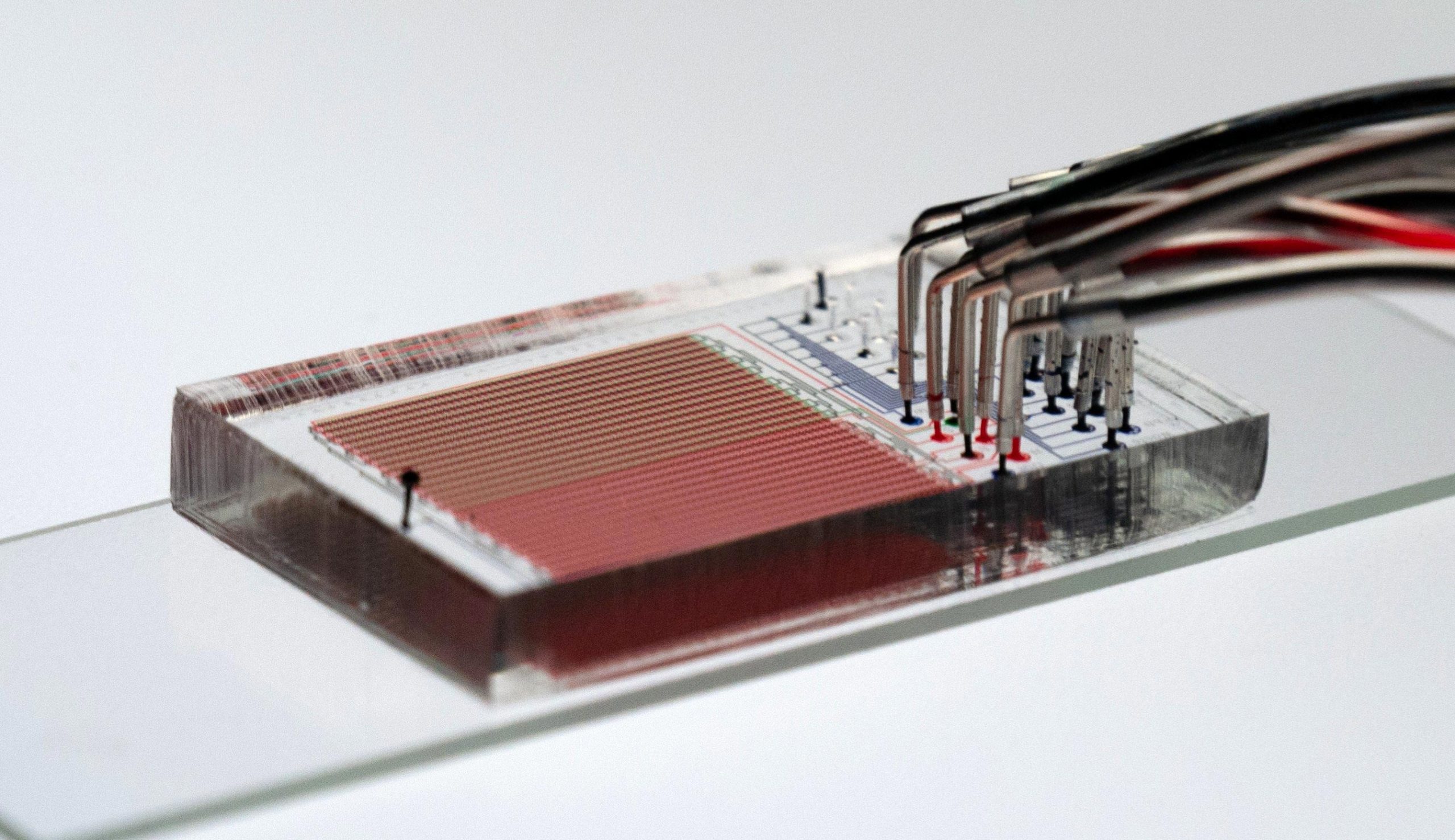
HT-MEK– short for High-Throughput Microfluidic Enzyme Kinetics– integrates microfluidics and cell-free protein synthesis technologies to drastically speed up the research study of enzymes. Credit: Daniel Mokhtari
A new tool that enables countless tiny experiments to run concurrently on a single polymer chip will let scientists study enzymes faster and more thoroughly than ever previously.
For much of human history, plants and animals were viewed to follow a various set of rules than remainder of the universe. In the 19th and 18th centuries, this culminated in a belief that living organisms were infused by a non-physical energy or “vital force” that allowed them to perform impressive transformations that could not be explained by standard chemistry or physics alone.
Researchers now understand that these transformations are powered by enzymes– protein particles comprised of chains of amino acids that act to speed up, or catalyze, the conversion of one kind of molecule (substrates) into another (items). In so doing, they enable responses such as food digestion and fermentation– and all of the chemical events that take place in each of our cells– that, left alone, would take place extraordinarily gradually.
Dubbed HT-MEK– short for High-Throughput Microfluidic Enzyme Kinetics– the method can compress years of work into just a couple of weeks by enabling thousands of enzyme experiments to be carried out all at once. HT-MEK integrates two existing innovations to rapidly speed up enzyme analysis. If commonly embraced, HT-MEK might not just enhance our fundamental understanding of enzyme function, however likewise catalyze advances in medication and market, the scientists say. HT-MEK might also accelerate a technique to drug advancement called allosteric targeting, which aims to increase drug uniqueness by targeting beyond an enzymes active website. Much further down the roadway, HT-MEK may even allow researchers to reverse-engineer enzymes and design bespoke ranges of their own.
” A chemical response that would take longer than the lifetime of the universe to occur on its own can happen in seconds with the help of enzymes,” stated Polly Fordyce, an assistant teacher of bioengineering and of genetics at Stanford University.
While much is now learnt about enzymes, including their structures and the chemical groups they use to help with reactions, the information surrounding how their forms link to their functions, and how they manage their biochemical wizardry with such remarkable speed and specificity are still not well understood.
A new technique, established by Fordyce and her coworkers at Stanford and detailed this week in the journal Science, could assist change that. Called HT-MEK– brief for High-Throughput Microfluidic Enzyme Kinetics– the technique can compress years of work into just a few weeks by making it possible for countless enzyme experiments to be performed all at once. “Limits in our ability to do sufficient experiments have avoided us from truly dissecting and comprehending enzymes,” said study co-leader Dan Herschlag, a professor of biochemistry at Stanfords School of Medicine.
Closeup picture of the HT-MEK gadget shows the individual nanoliter-sized chambers where enzyme experiments are performed. Credit: Daniel Mokhtari
By enabling researchers to deeply probe beyond the small “active website” of an enzyme where substrate binding occurs, HT-MEK might expose ideas about how even the most remote parts of enzymes work together to accomplish their remarkable reactivity.
” Its like were now taking a flashlight and rather of simply shining it on the active website were shining it over the whole enzyme,” Fordyce said. “When we did this, we saw a lot of things we didnt expect.”
Enzymatic tricks
HT-MEK is developed to change a tiresome procedure for purifying enzymes that has traditionally involved engineering bacteria to produce a specific enzyme, growing them in large beakers, breaking open the microbes and after that separating the enzyme of interest from all the other unwanted cellular elements. To piece together how an enzyme works, scientists present deliberate errors into its DNA plan and then evaluate how these mutations affect catalysis.
This procedure is pricey and time consuming, however, so like an audience raptly focused on the hands of a magician during a conjuring technique, scientists have mostly restricted their scientific examinations to the active websites of enzymes. “We understand a lot about the part of the enzyme where the chemistry takes place due to the fact that individuals have made mutations there to see what occurs. Thats taken decades,” Fordyce said.
However as any connoisseur of magic techniques understands, the key to a successful impression can lie not just in the actions of the magicians fingers, but may also include the deft positioning of an arm or the torso, a misdirecting patter or discrete actions happening unofficial, invisible to the audience. HT-MEK allows researchers to quickly move their look to parts of the enzyme beyond the active website and to explore how, for instance, changing the shape of an enzymes surface may affect the operations of its interior.
” We ultimately would like to do enzymatic tricks ourselves,” Fordyce said. “But the initial step is determining how its done prior to we can teach ourselves to do it.”
Enzyme experiments on a chip
The innovation behind HT-MEK was developed and fine-tuned over 6 years through a partnership between the labs of Fordyce and Herschlag. “This is a remarkable case of engineering and enzymology coming together to– we hope– revolutionize a field,” Herschlag said. “This job went beyond your common collaboration– it was a group of people working collectively to fix a really difficult problem– and continues with the methods in location to try to respond to tough concerns.”
HT-MEK integrates two existing innovations to rapidly speed up enzyme analysis. “Microfluidics diminishes the physical space to do these fluidic experiments in the same method that integrated circuits decreased the real estate needed for computing,” Fordyce said.
The 2nd is cell-free protein synthesis, a technology that takes just those essential pieces of biological equipment needed for protein production and integrates them into a slushy extract that can be utilized to develop enzymes artificially, without needing living cells to work as incubators.
” Weve automated it so that we can utilize printers to deposit microscopic spots of synthetic DNA coding for the enzyme that we desire onto a slide and then align nanoliter-sized chambers filled with the protein starter mix over the spots,” Fordyce explained.
The researchers used HT-MEK to study how mutations to different parts of a well-studied enzyme called PafA impacted its catalytic ability. Credit: Daniel Mokhtari
Due to the fact that each small chamber contains only a thousandth of a millionth of a liter of material, the scientists can engineer countless versions of an enzyme in a single gadget and study them in parallel. By tweaking the DNA guidelines in each chamber, they can customize the chains of amino acid particles that make up the enzyme. In this way, its possible to systematically study how various modifications to an enzyme affects its folding, catalytic capability and capability to bind other proteins and little molecules.
When the team applied their method to a well-studied enzyme called PafA, they discovered that anomalies well beyond the active website impacted its capability to catalyze chemical responses– certainly, the majority of the amino acids, or “residues,” making up the enzyme had effects.
The scientists also discovered that a surprising number of anomalies triggered PafA to misfold into an alternate state that was unable to perform catalysis. “Biochemists have known for decades that misfolding can occur but its been extremely difficult to determine these cases and much more difficult to quantitatively approximate the quantity of this misfolded things,” said study co-first author Craig Markin, a research study researcher with joint consultations in the Fordyce and Herschlag laboratories.
” This is one enzyme out of thousands and thousands,” Herschlag highlighted. “We anticipate there to be more discoveries and more surprises.”
Accelerating advances
If extensively embraced, HT-MEK might not just enhance our standard understanding of enzyme function, however also catalyze advances in medicine and market, the researchers say. “A lot of the commercial chemicals we use now are bad for the environment and are not sustainable. However enzymes work most efficiently in the most environmentally benign compound we have– water,” said study co-first author Daniel Mokhtari, a Stanford graduate trainee in the Herschlag and Fordyce labs.
HT-MEK might also speed up a technique to drug advancement called allosteric targeting, which intends to increase drug uniqueness by targeting beyond an enzymes active website. Enzymes are popular pharmaceutical targets due to the fact that of the key function they play in biological processes. However some are thought about “undruggable” because they come from families of associated enzymes that share the exact same or extremely comparable active websites, and targeting them can lead to negative effects. The concept behind allosteric targeting is to create drugs that can bind to parts of enzymes that tend to be more separated, like their surface areas, but still control particular elements of catalysis. “With PafA, we saw functional connectivity in between the surface and the active site, so that offers us hope that other enzymes will have comparable targets,” Markin said. “If we can identify where allosteric targets are, then well be able to start on the more difficult job of actually developing drugs for them.”
The sheer quantity of information that HT-MEK is expected to create will also be a benefit to computational techniques and device knowing algorithms, like the Google-funded AlphaFold task, created to deduce an enzymes complex 3D shape from its amino acid series alone. “If artificial intelligence is to have any possibility of accurately predicting enzyme function, it will require the sort of information HT-MEK can offer to train on,” Mokhtari said.
Much further down the road, HT-MEK might even allow researchers to reverse-engineer enzymes and design bespoke ranges of their own. If it were actually real that the only part of an enzyme that matters is its active website, then we d be able to do that and more currently.
Herschlag hopes that adoption of HT-MEK among researchers will be quick. “This is a tool that has the possible to supplant conventional approaches for an entire community.”
Referral: “Revealing enzyme practical architecture via high-throughput microfluidic enzyme kinetics” by C. J. Markin, D. A. Mokhtari, F. Sunden, M. J. Appel, E. Akiva, S. A. Longwell, C. Sabatti, D. Herschlag and P. M. Fordyce, 23 July 2021, Science.DOI: 10.1126/ science.abf8761.
Fordyce belongs to Stanford Bio-X and the Wu Tsai Neurosciences Institute, and an executive committee member of Stanford ChEM-H. Herschlag is member of Bio-X and the Stanford Cancer Institute, and a professors fellow of ChEM-H. Other Stanford co-authors include Fanny Sunden, Mason Appel, Eyal Akiva, Scott Longwell, and Chiara Sabatti.
The research was moneyed by Stanford Bio-X, Stanford ChEM-H, the Stanford Medical Scientist Training Program, the National Institutes of Health, the Joint Initiative for Metrology in Biology, the Gordon and Betty Moore Foundation, the Alfred P. Sloan Foundation, the Chan Zuckerberg Biohub, and the Canadian Institutes of Health Research.