Happify is rolling out a digital healing developed to deal with anxiety and anxiety. Picture credit: Happify Health
Happify designed Ensemble using information from people who have utilized itss app over the last 8 years.
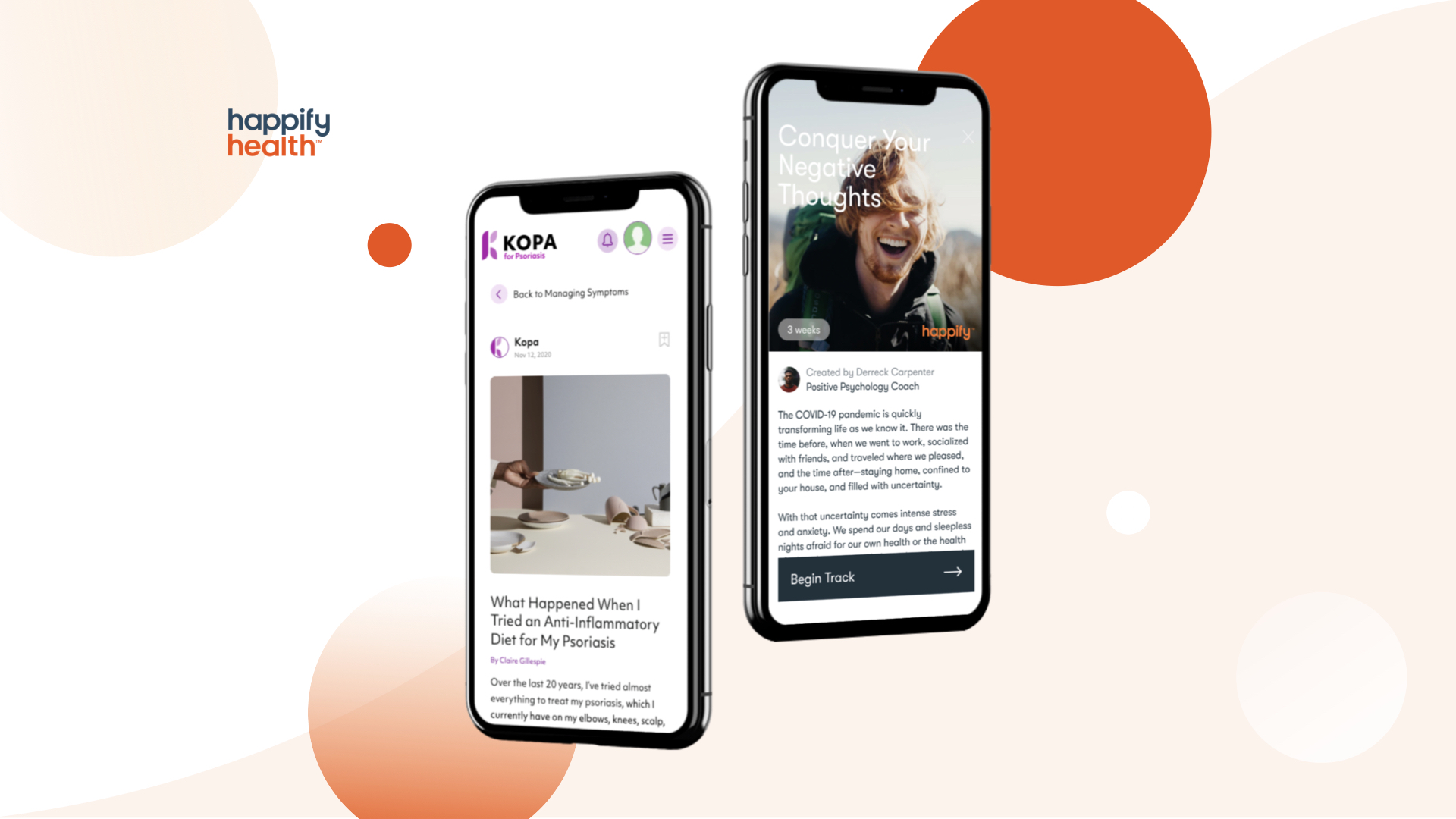
Happify is presenting a digital restorative created to treat stress and anxiety and anxiety. Its not yet FDA-cleared, however the business is making it readily available through a regulative modification implemented by the Food and Drug Administration during the pandemic. Photo credit: Happify Health
After the Food and Drug Administration loosened up standards in 2015 for digital health products treating some behavioral health conditions, startup Happify Health is making an app-based treatment for stress and anxiety and anxiety offered to clients.
The companys new digital restorative, called Ensemble, uses app-based exercises to assist individuals change negative attitude patterns and develop healthy habits. Its not FDA cleared, and is only available by prescription or through an investigational study, which Happify wants to use to get data for a future regulatory submission.
New York-based Happify already built an app that utilizes activities and video games to assist individuals with unfavorable thoughts and stress. It raised $73 million in financing earlier this year, and has struck collaborations with numerous health plans and pharmaceutical business.
Happify created Ensemble utilizing data from people who have used itss app over the last eight years. Individuals who take part in the study will utilize the app for 10-weeks, completing two 15- to 30-minute workouts each day, while guided by a chatbot. Their progress is shared with their physician through a dashboard.
Dr. Murray Zucker, Happifys chief medical officer, said in a news release that the activities were developed to target underlying processes that prevail to both anxiety and stress and anxiety. Although the app is supposed to assist with symptoms, its not meant to change medication or treatment.
Happify is among a plethora of mental health apps that have appeared in the last few years, and among a handful of business that have launched software-based treatments in the in 2015 under the FDAs brand-new guidance.
In April of 2020, the firm chose that throughout the Covid-19 emergency, designers might release “electronic behavior modification gadgets” and apps to treat psychiatric conditions without looking for 510(k) clearance. Its not yet clear how business who have made products available will navigate this after the general public health emergency situation ends.